Streamline and Improve
Assay Workflow and Analysis
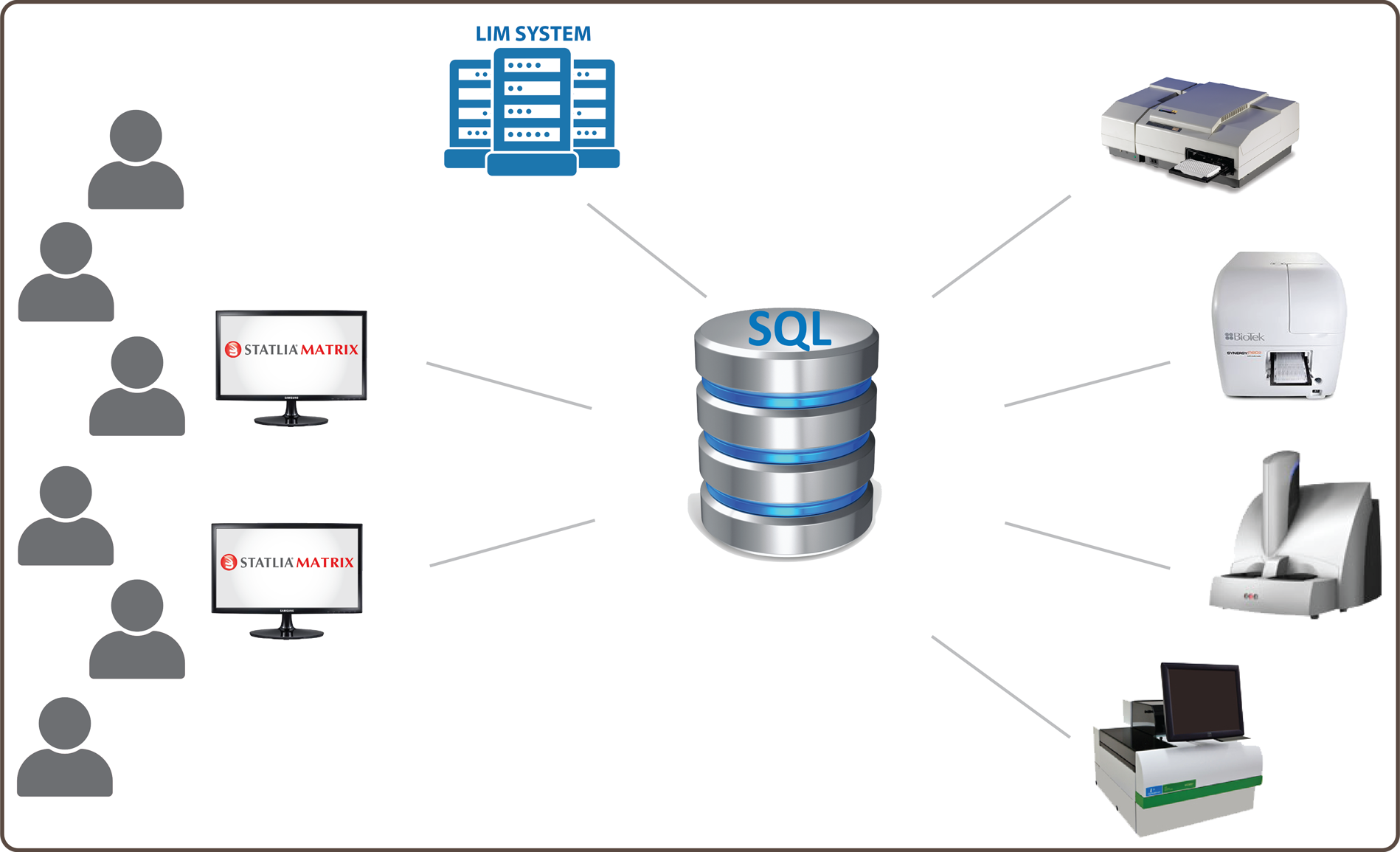
Integrate multiple users, instruments, desktops (virtual or PC), and a secure Microsoft SQL Server database on one STATLIA MATRIX software system for all of your laboratory’s types of assay techonologies: ELISA/LBA, potency bioassay, and immunogenicity.
STATLIA MATRIX 5.0 is a GxP enterprise software designed to streamline assay workflow, enhance analysis capabilities, and reduce manual documentation for multiple testing technologies. Designed to integrate with any large or small
laboratory, STATLIA MATRIX is structured around a robust Microsoft SQL Server database that secures, organizes, and provides easy access to all of your assays, raw data, assay data, reports, approvals, and more.
laboratory, STATLIA MATRIX is structured around a robust Microsoft SQL Server database that secures, organizes, and provides easy access to all of your assays, raw data, assay data, reports, approvals, and more.
Interface as few or as many instruments as needed with multiple users and one or more desktops (virtual or PC) on one standardized STATLIA MATRIX software system to streamline your assay workflow and analysis.
- One system for ELISA/LBA, Potency Bioassay, IMG assays
- Interface multiple instruments from different manufacturers
- 3 potency assay methods: Equivalence, Chi-Square, F Test
- Automate assay/unknown Pass/Fails based on your SOPs
- Gold standard 5PL and 4PL with advanced analysis
- All assay data secured and accessible in MSSQL database
- Follows GAMP 5 and enables 21 CFR Part 11 compliance
- Automated IQ/OQ Validation Package
- Track reagents and controls used in each assay
Reagent and Control Inventory