Run Test Method Validation Reports in Minutes for FDA Submissions
STATLIA MATRIX can generate a Test Method Validation Report in minutes following the 2018 FDA’s Bioanalytical Method Validation Guidance for Industry. Select, organize and analyze a Test Method Validation Report with one click. Just run your method validation assays, then select the assays and click the Method Validation button. All of the assays and data are grouped and analyzed into a single report for method validation submissions.

Get a Test Method Validation Report – With One Click
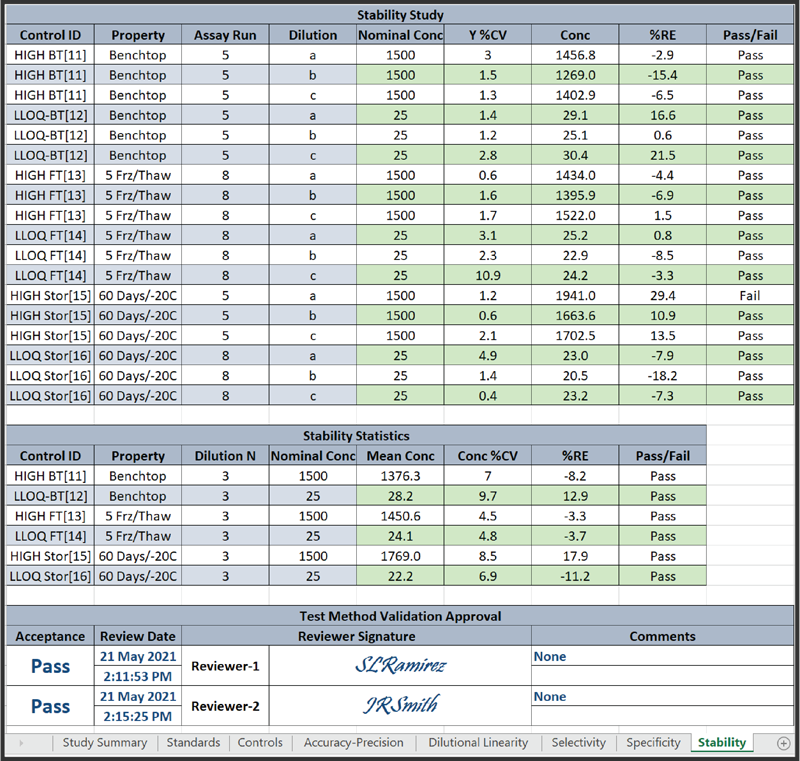
The Stability page above, one of eight method validation report pages, includes all of the controls run in different assays with stability conditions, such as 5 Freeze/Thaw cycles and 60 Days/-20C. Each page includes the %RE Pass/Fail results set by the laboratory for each control.
Select all of the assays to include then click the button. It is that easy to generate a report for method validation submissions. You can also create quality assurance reports to analyze a few or hundreds of your previously run assays.