Multiplex Assay Software
Detailed, Informative Analysis Provides More Insight to Analyze, Optimize, Manage Your Multiplex AssaysSee your data in STATLIA MATRIX.
STATLIA MATRIX MP Expands Analysis, Optimization, Data Management Options for Your Multiplex Assays
Complete multiplex projects faster with more accurate results and detailed metrics to make reliable determinations and optimizations of analytes in a multiplex assay in less time. STATLIA MATRIX computes immunoassay, potency, and immunogenicity multiplex assays.
- Easy, intuitive interface to run, analyze, manage, and optimize multiplex assays
- Dashboard Pass/Fail summary for each analyte in a plex with QC metrics based on your SOP criteria
- Limits of Quantitation (LOQs) and Limits of Detection (LODs) computed for each analyte
- Gold standard weighted 5PL, 4PL curve fitting improves accuracy, reliability
- Outlier masking (auto or manual)
- Auto masking option masks nonmonotonic hooks in curve fits
- Import worklist with unknown identifications, export results to LIM System
- Auto suppress failed results or failed unknowns before accepting results
- Auto compute QC ranges, limits, and more from pooled previous panel runs
- % Error computed for each result to measure its reliability
- Optimization tools to enhance performance of each analyte
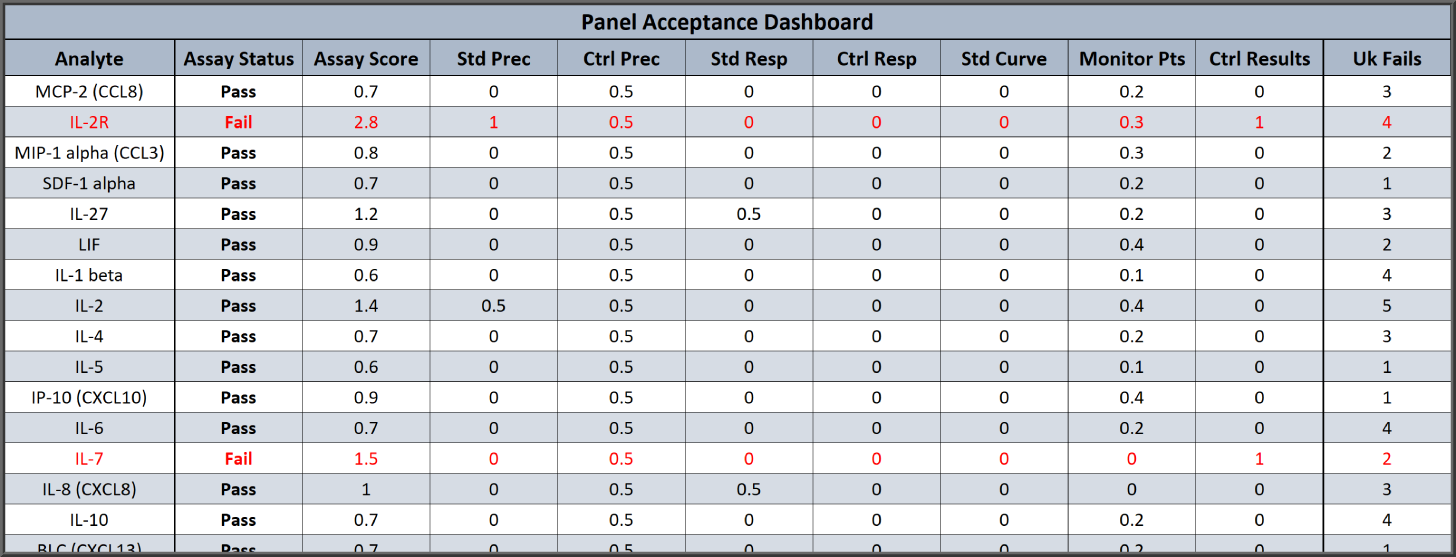
Dashboard Summary of Each Analyte with Pass/Fail and QC Metrics for Quick, Detailed Analysis of Panel Run
Each multiplex run can include the summary table above for a quick review of the Pass/Fail determinations for each analyte and its unknowns based on your criteria. The table also includes a summary of the different QC metrics you entered which can be the same or different for each analyte, such as Standard Precision (%CV), Standard %Bias (backfit recovery), Curve Fit threshold, Control Results , and more. Any flagged metrics are listed at the bottom of the summary report.
With the custom report editor and the QC Acceptance function, you can modify the summary report and Panel Acceptance Summary table to match your laboratory’s Pass/Fail criteria and the QC metrics that you follow. The report editor also allows you to customize the entire summary report as well as the optional individual reports for each analyte in the multiplex.
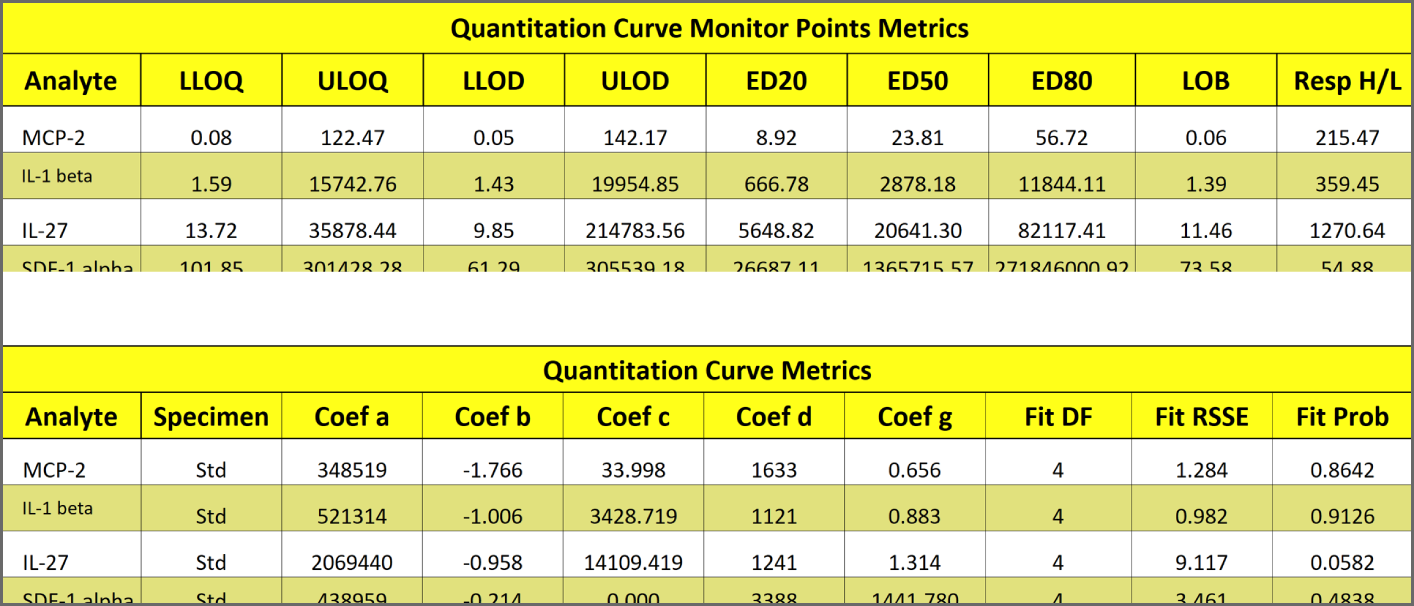
LOQs, LODs and Other Monitor Points Computed for Each Analyte from the Standard Curve Data
The upper and lower Limits of Quantitation (LOQs) and Limits of Detection (LODs) are automatically computed for each analyte in each multiplex run. The Estimated Doses (ED20, ED50, ED80) Limits of Blank (LOB) and curve length (Response High/Low or H-L or Asymptote H/L or H-L) are also computed. For more accuracy, STATLIA MATRIX also computes these metrics from a pool of previous runs of the same multiplex.
The LOQs determine the reportable range of reliable results. The LODs determine the range that the instrument can detect a reliable signal for each analyte. The LOQs and LODs are computed for each analyte in a multiplex run from their standard curve data. No extra samples need to be run to compute or verify the LOQs or LODs. See the Tech Note: LOQs, LODs, Precision Error Profile, and Reportable Range and manuscript Determining the Error of Dose Estimates and Minimum and Maximum Acceptable Concentrations from Assays with Nonlinear Dose-Response Curves.
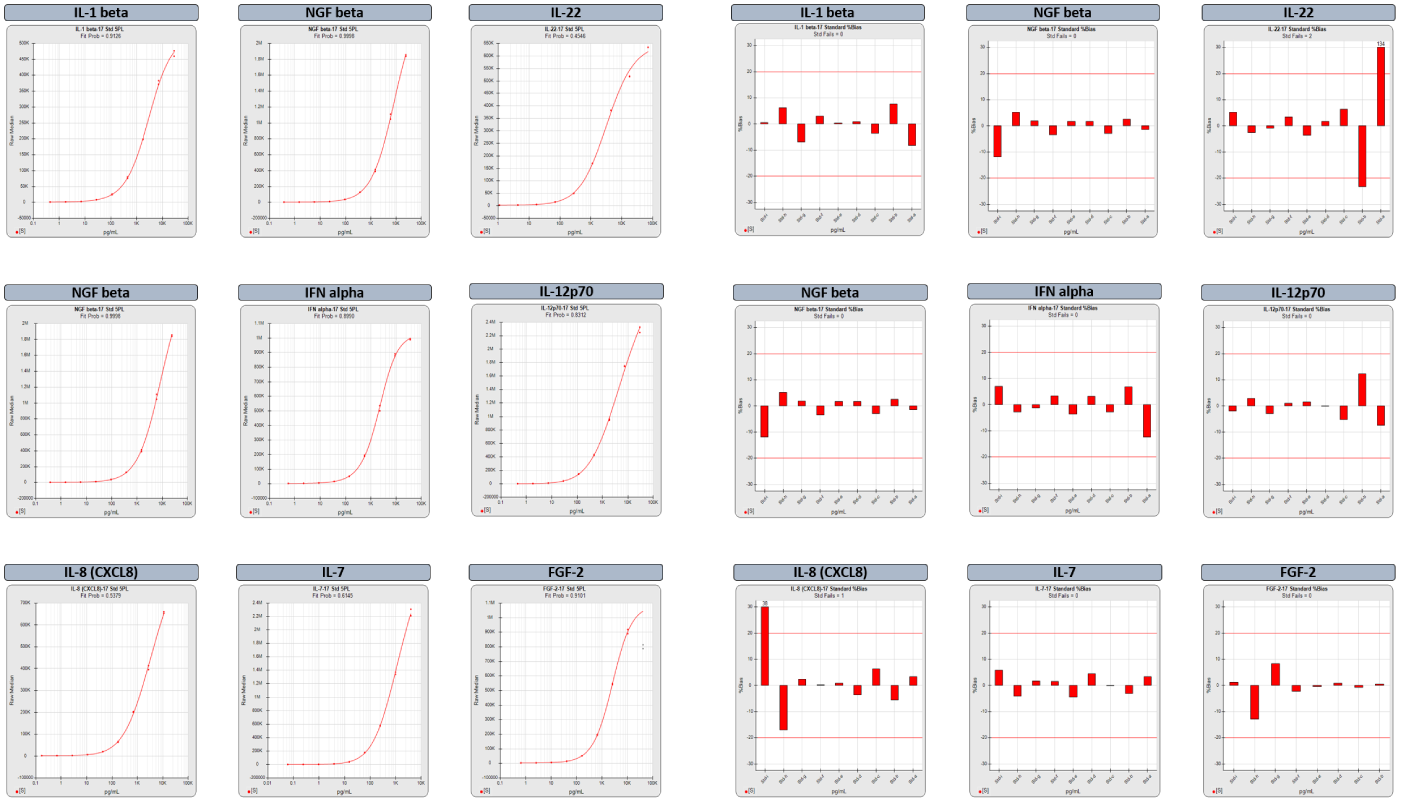
Powerful Graphs Offer Quick Insight into Performance and Reliability of Each Analyte in a Multiplex Run
For the summary report of a multiplex run, select from among multiple thumbnail graphs that provide a quick overview of the performance of each analyte in a plex and its reliability for accurate results.
The two graph options above include the standard curves and the backfit recovery %Bias (red bars) of each standard dilution. The %Bias is the difference between the computed and expected concentrations of each standard point. The %Bias graphs show at a glance which regions of the curve have acceptable bias. The graphs for the FGF-2 analyte were computed after the software automatically masked the highest dilution to remove the hook. The masked responses are grayed out on the standard curve graph.
With the report editor, create ifferent summary and analyte reports for different multiplex panels. STATLIA MATRIX includes the following graph options for each analyte in a multiplex summary report and for the individual assay report of each analyte:
- Standard Curves
- %Bias Backfit Recovery
- Control Trending (LJ) Charts
- Control Results (Dose and Response)
- Standard Responses Confidence Limits
- LOQs and %Error of Each Unknown
- Squared Residual Error of Each Standard Dilution
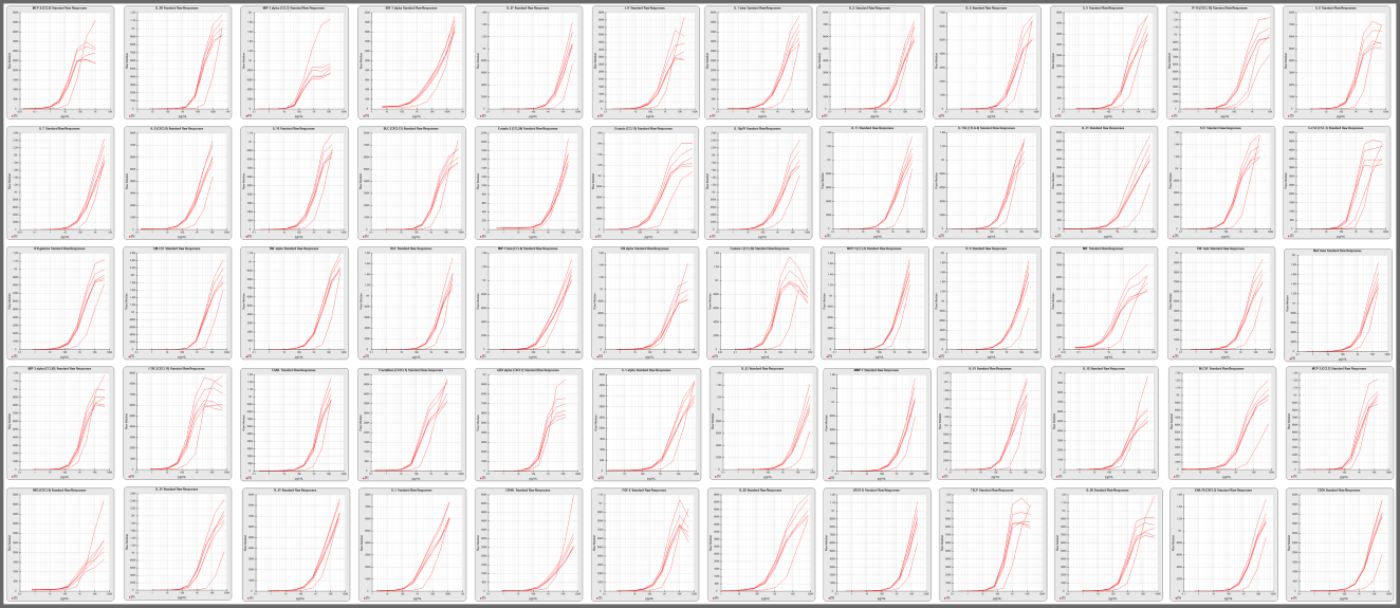
Pooled Panel Runs Provide Detailed Analysis to Optimize, Validate and Monitor Multiplex Assays
STATLIA MATRIX can pool previous multiplex panel runs from the same multiplex to compute more accurate weighting, control ranges, confidence limits, and other performance metrics and graphs for each analyte in the multiplex. These computations can be computed automatically as you process your panel runs or by just selecting the desired runs and clicking compute in the software’s QA function. This feature and analysis is especially helpful for comparing new lot performance in a multiplex.
The software also computes several graphs and metrics that can be used to optimize each analyte in a multiplex. The pooled previous runs make it much easier to analyze performance and identify issues that can then be modified to improve the performance and reliability of an analyte.
Insightful Analysis, Parallelism, Automated Masking and More Offer Useful Tools to Optimize Analytes
STATLIA MATRIX provides many helpful analysis graphs, metrics and testing technologies that can provide invaluable information to assess, optimize, and analyze each analyte in a multiplex.
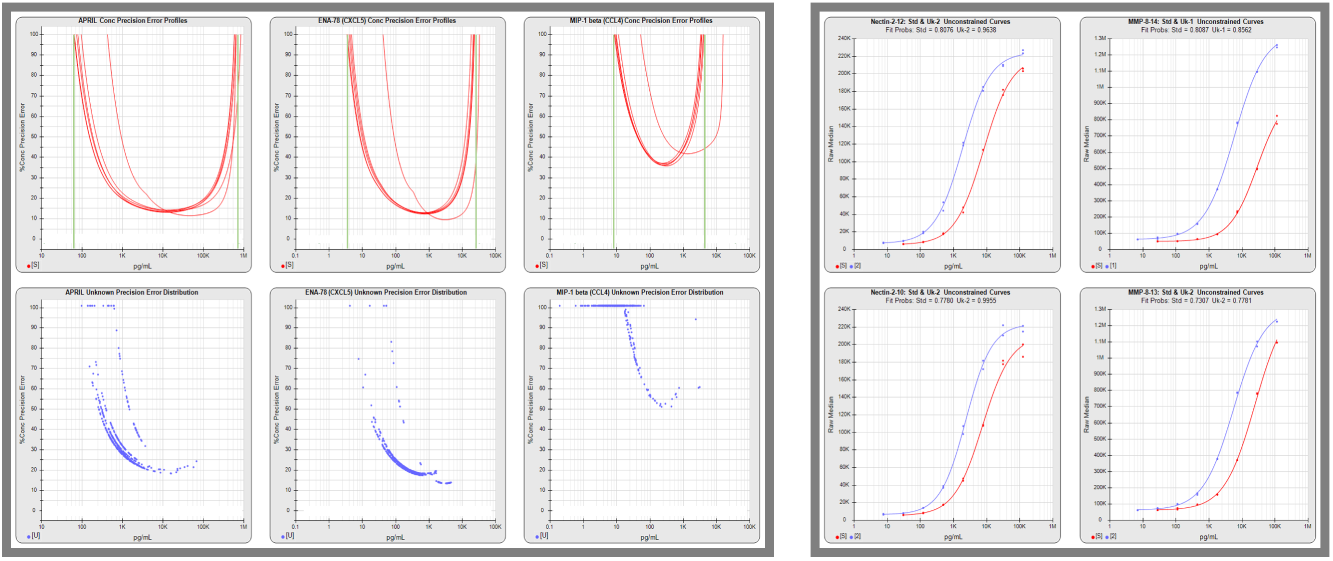
The graphs above on the left show the standard curve error profiles (red lines) from 6 panel runs with the %Error (y-axis) of the results throughout the concentration range (x-axis). The “U” shape reflects the lowest errors in the middle of the standard curves with the %Error increasing toward the ends of the curve. The green lines show the limits of quantitation for each analyte.
The lower graphs show the unknown concentrations plotted at their %Errors (blue dots) from 6 runs and highlight the focus for each analyte’s optimization. These graphs also plot the reliability of the results. For example, an unknown with a result of 100 pg/mL and a 20 %Error means it is 95% likely that the true concentration is between 80-120 pg/mL.
The parallelism multiplex assay on the right can be computed with one of three relative potency methodologies referenced in the literature and regulatory guidelines: RSSE Chi-Square, Finney’s F Test, or Equivalence. Among other uses, multiplex potency assays can be used to determine the relative potency between lots.
If the Mask Nonmonotonic Dilutions box is checked, STATLIA MATRIX will automatically mask each unacceptable dilution at the high end of the curve when the multiplex is computed. Users can also pre-select dilutions to be masked at each end of the curve. Precision and residual outliers are also automatically masked if selected. Any masked samples will be excluded from the computations, but their responses will still be included in the reports and color coded to show they were masked.
Easily Analyze Multiplex (or Singleplex) Assays
Select New Panel (Multiplex) or New Assay (Singleplex)
All Data and Settings Saved in Database for Each Multiplex Run
Import a raw data file and click compute. The software does the rest. Summary report provides an informative overview of each analyte’s performance for quick review. Export results to LIM system. Review additional analysis of each panel or analyte as needed with customized reports. Detailed analysis of each analyte with all data, analysis, and results easily accessible from the software’s Microsoft SQL Server database.