STATLIA MATRIX 5.0
Enterprise GxP Software for Potency Bioassay, Immunoassay, and Immunogenicity AnalysisBrendan Bioanalytics Virtual Booth
BEBPA 2021 US Bioassay Conference, March 22 – 25
Thank you for visiting our virtual booth for the 2021 BEBPA US Bioassay Conference. As a sponsor of this important conference, we invite you to see some of the advanced capabilities that our new software STATLIA MATRIX 5.0 offers to significantly improve the analysis and workflow efficiencies for running, analyzing, managing and validating Potency Bioassays, LBA / ELISA Immunoassays, and Immunogenicity Assays.
Learn more about STATLIA MATRIX by attending our BEBPA conference presentation and from our website, www.Brendan.com
BEBPA VIRTUAL CONFERENCE PRESENTATION
Advanced Analysis for Potency, LBA, IMG Assays with New STATLIA MATRIX 5.0 Software
Virtual Presentation
Wednesday, March 24, 2021
11:20 am – noon PDT
Wednesday, March 24, 2021
11:20 am – noon PDT
Presentation Highlights:
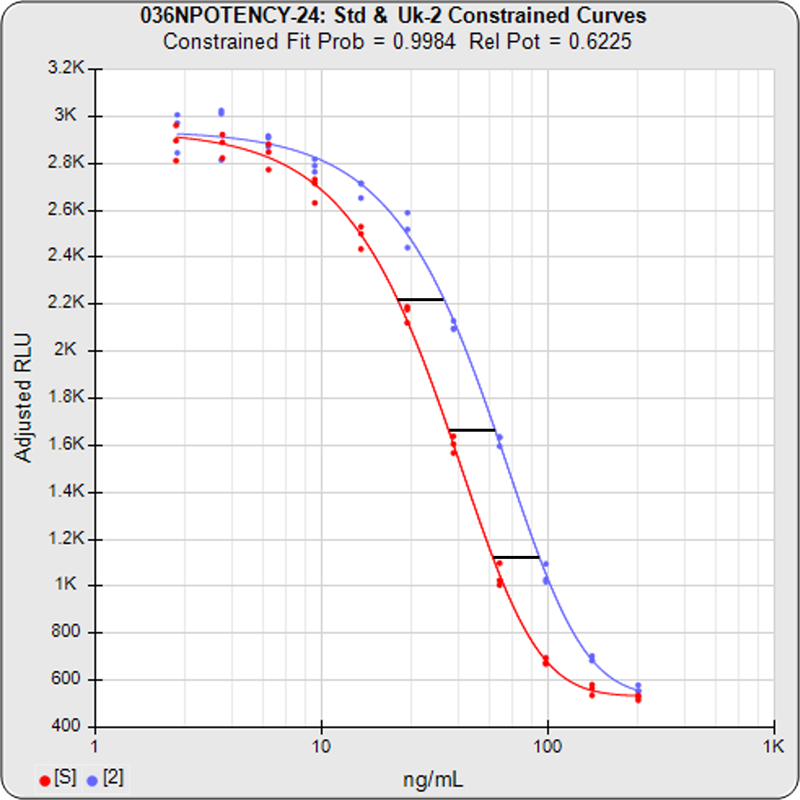
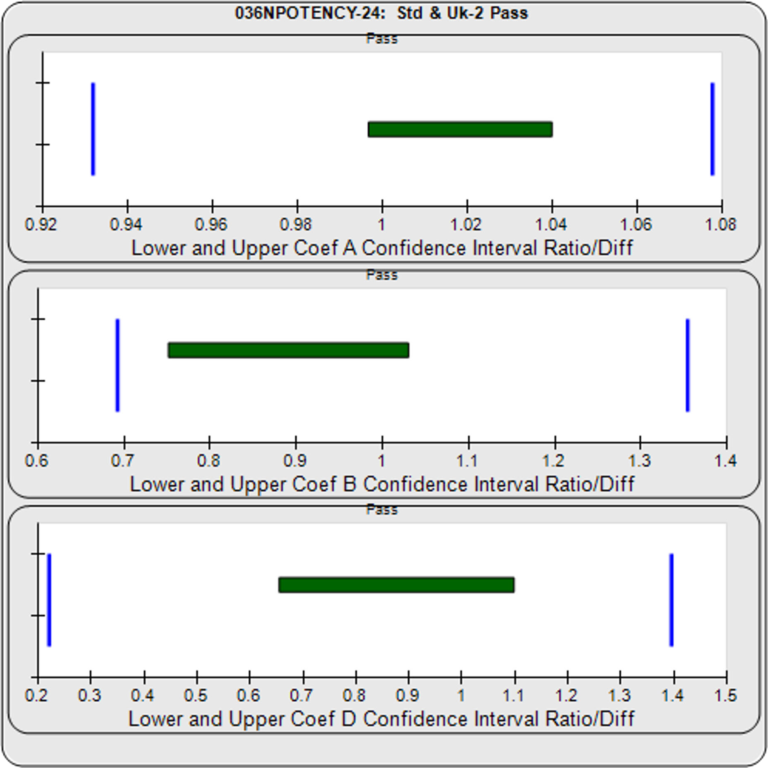
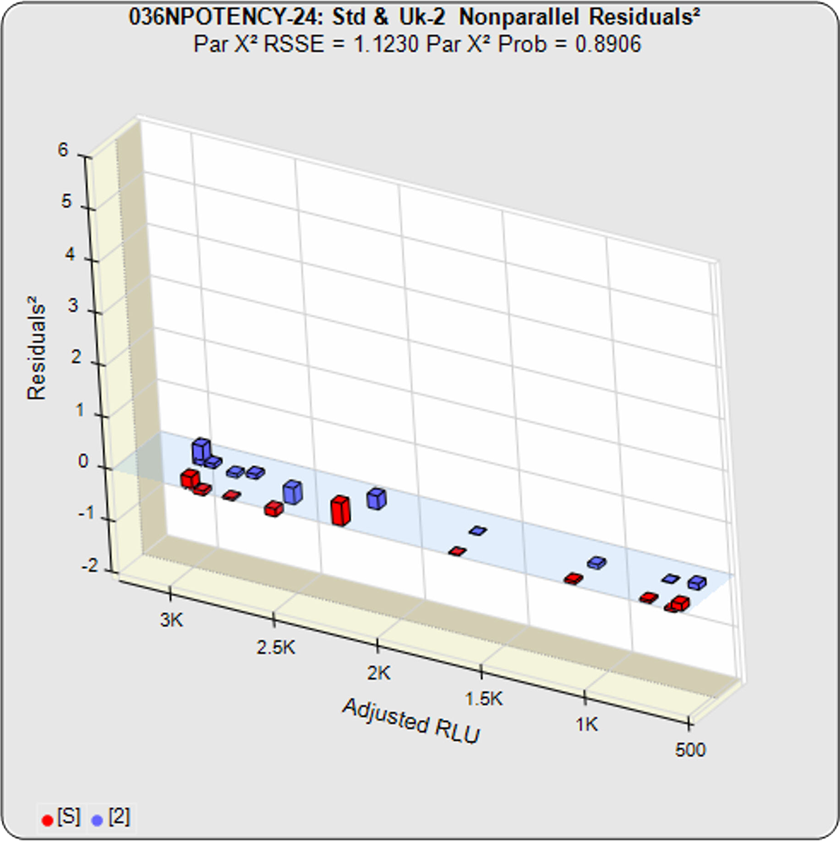
- 3 Potency Methods from USP 1034 (Equivalence, Chi-Square, and F Test)
- Handles difficult assays (lack of plateaus, hooks, asymmetry)
- Determines boundary goalposts, thresholds, and other metric ranges from pooled assays
- Tools to optimize dilution doses and other factors
- Auto Pass/Fail based on your SOPs
- Auto IQ/OQ Validation Package

Speaker:
John R Dunn II, Ph.D.
CTO, Brendan Bioanalytics

STATLIA MATRIX Technologies
- Potency Bioassay Methods
- Equivalence Method
- Chi-Square Method
- F test
- LBA / ELISA / Immunoassays
- Immunogenicity Assays
Advanced Analysis and Workflow Efficiencies
- New graphs and metrics improve and simplify analysis
- MSSQL database secures and tracks all assay data
- Custom reports put only the data you want at your fingertips
- All assay data and reports organized and accessible
- Gold standard 5PL and 4PL improve analysis
- Auto outlier rejection
- LOQs and LODs computed each assay and for test
- PK Method Validation Reports
- Analysis tools to optimize dilution doses and other factors