Brendan Bioanalytics develops advanced analysis and workflow software for multiplex and singleplex immunoassay, potency bioassay/parallelism, and Immunogenicity/qualitative assays.

The new STATLIA MATRIX MP version provides detailed multiplex analysis of each analyte that gives you more control, insight, and options to run, analyze, develop, optimize, and manage your multiplex assays and data.

Advanced analysis and workflow software for multiplex and singleplex assays.
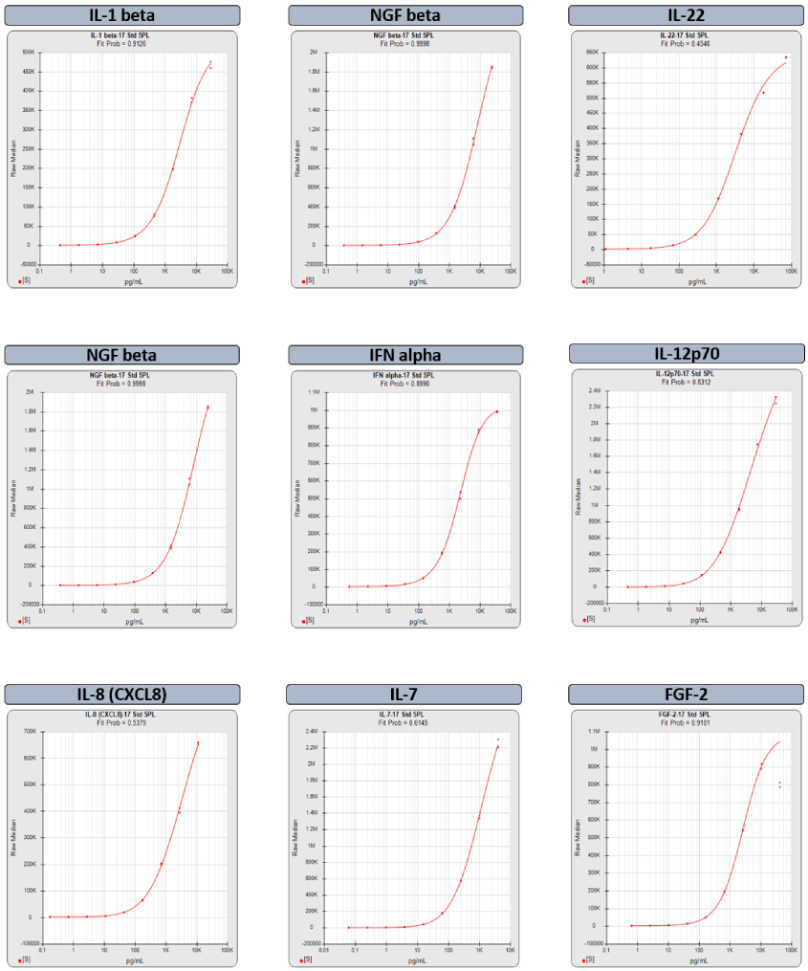
Multiplex Assay Analysis
Watch the Video
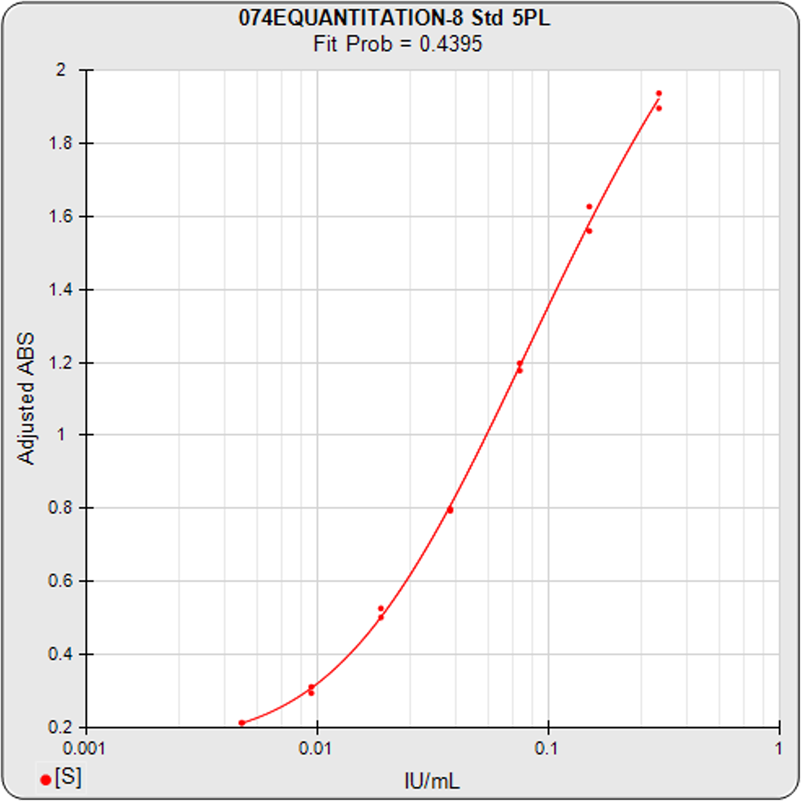
Singleplex Assay Analysis
Watch the Video
Advanced Analysis.
Gold standard 5PL and 4PL curve fitting models with complete selection of robust metrics, graphs, computations and analysis provide accurate and reliable results every multiplex or singleplex assay. Software automatically computes weighting, control ranges, limits, goalposts, thresholds, and more from your pooled previous runs.

Simplified Analysis.
Easily run, analyze, develop, optimize, and manage all multiplex or singleplex assay runs and reports with all data safely accessible in a secure Microsoft SQL Server database. Review individual or pooled previous runs with a complete statistical analysis. Reagent tracking, custom reports, outlier masking, and more are always ready for your next assay.
Scalable Analysis.
Interface as many users, desktops (physical or virtual), multiplex instruments and microplates from different manufactures as needed into one standardized system on your LAN, cloud or Citrix environment. Import worklists and export results to your LIM system for streamlined workflow with complete regulatory compliance.
Powerful Computational Functionality For Your Multiplex and Singleplex Assays
Multiplex Assays
STATLIA MATRIX MP provides detailed, summary analysis of your multiplex assay performance and reliability with informative graphs and metrics of each analyte and easy access to all your data and reports. Customize analysis with custom reports and QC Pass/Fail Acceptance for each analyte based on your SOP criteria.
ELISA/LBA Assays
STATLIA MATRIX provides a comprehensive software program with the advanced computations and analysis to compute all immunoassay quantitation technologies, including ELISA, EIA, FIA, LIA, ECL, RIA, and Label-Free tests. The software also computes immunogenicity Tier 1, Tier 2, and Tier 3 tests plus methodologies for determining cut point factors.
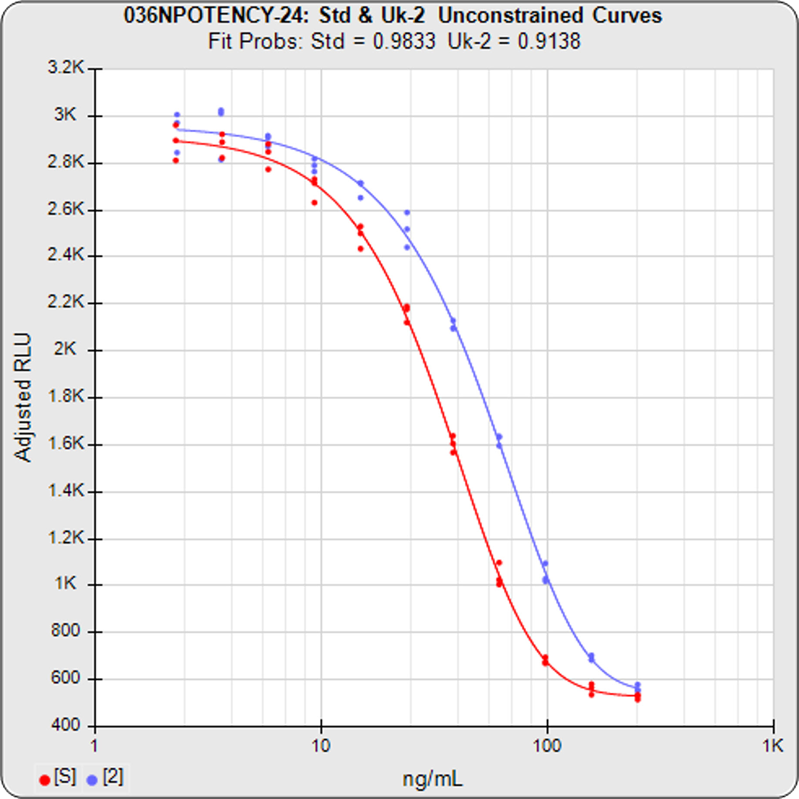
Potency Assays
STATLIA MATRIX offers accurate and reliable analytical methodologies for implementing either of the three parallelism methods referenced in the regulatory guidelines for potency assays: Equivalence Method (empirical test), RSSE Chi-Square Method (direct parallelism measure), and F Test (hypothesis test).
Dashboard Summary with Pass/Fail
Software Tools to Simplify and Optimize Running and Analyzing Your Assays
Assay Development
Insightful graphs and metrics enable you to determine optimal dilution doses, a usable dose range, expand the limits of quantitation, and make many other significant refinements to improve your analyte or test performance in your multiplex or singleplex assays.
QC Acceptance
Select your precision, response, and data reduction metrics and set your acceptance criteria for the automatic Pass/Fail determinations of your assay and each unknown, based on your SOP’s.
Reports
View the software’s informative analysis in: Summary and Analyte Reports (customizable) for multiplex assays, Assay Reports for singleplex assays (customizable), Performance Analysis reports for analysis and behavior of the test method or analyte, and Quality Assurance reports for analysis of a group of previous multiplex or singleplex assays you select.

Method Validation
Generate a Method Validation Report following the FDA’s guidelines for accuracy-precision, dilutional linearity, selectivity, specificity, and stability tests. With one click, all the controls and assays used to analyze these test protocols are formatted in one Excel spreadsheet.
Automated IQ/OQ Validation
The STATLIA MATRIX Auto IQ/OQ Validation Package provides all of the components required for a complete validation of the software and detector interfaces. The validation program auto-executes in about 30 minutes and the completed validation report is then ready for signatures.
Regulatory Compliance
STATLIA MATRIX is a GAMP-5 software package that enables complete 21 CFR Part 11 regulatory compliance for your GxP bioanalytical laboratory. Electronic signature, event log audit trail, security group permissions, secure data integrity, and more are provided.
Some of Our STATLIA Customers